Introduction
Do you know? The brain, as the "central processor" of the human body, operates efficiently with low energy consumption, a feat that even artificial intelligence struggles to match. Disruptions in its energy regulation are also a risk factor for neurological diseases. The research team led by Professor Ma Huan, published in Science, uses multidisciplinary approaches to offer new insights into how the brain "conserves energy" and combats cognitive aging. Let’s take a closer look!
On December 20, 2024, Professor Ma Huan's research team from Zhejiang University's State Key Lab of Brain-Machine Intelligence and School of Medicine published a paper in Sciencetitled "Boosting neuronal activity-driven mitochondrial DNA transcription improves cognition in aged mice." This study, using a multidisciplinary approach, explores the relationship between brain bioenergy plasticity regulation and cognitive aging, offering a new perspective and theoretical framework for understanding the brain's "energy-saving" mechanisms and combating cognitive decline. It also reveals the brain's efficient, low-energy operating mechanisms, providing fresh insights and directions for artificial intelligence to enhance information processing while reducing energy consumption.

As the core organ responsible for cognition and consciousness, the brain requires a significant amount of biological energy to maintain key functions such as learning, memory, and emotional regulation. To enable the brain to efficiently utilize energy, living organisms must finely regulate this process, achieving the parallel processing and storage of vast amounts of information with low energy consumption. This energy-efficient characteristic is the target of supercomputers and artificial intelligence technologies, representing a pinnacle that human technology has yet to reach. At the same time, brain energy regulation is closely linked to human health, with imbalances being considered a key risk factor for neurological diseases, particularly age-related neurodegenerative conditions.Both the energy shortage issues triggered by high energy consumption in AI development and the rapidly growing elderly population in aging societies present significant challenges that humanity must face in the modern era. From a scientific perspective, understanding how the mammalian brain integrates the fundamental elements of energy, matter, and information—the building blocks of life—offers not only a roadmap for imitating and even surpassing the brain’s "low-energy, high-efficiency" capabilities formed over long periods of evolution, but also an important opportunity to explore solutions to aging-related issues. In response to this critical frontier in neuroscience, Professor Ma Huan's research team has conducted in-depth studies.
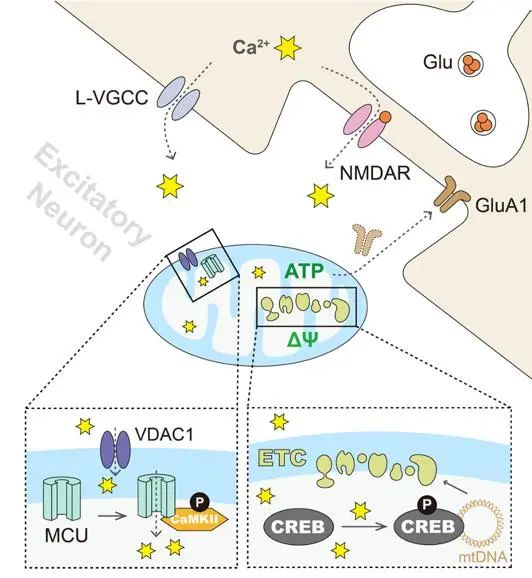
Figure 1: Schematic of the E-TCmito mechanism.
During information processing and storage, the brain's unique characteristic is that the strength of the connections between neurons can be dynamically adjusted based on activity and experience. This maintenance of neural plasticity requires neurons to regulate gene transcription in the cell nucleus via neural activity, enabling the synthesis of new genes and proteins—fundamental processes for cognitive functions like learning and memory. Mitochondria, as the primary energy providers in cells, are the only organelles outside the nucleus in mammals that possess their own genome. Their gene transcription is crucial for mitochondrial biogenesis and the energy supply to the body. So, during information processing, can neural activity regulate mitochondrial gene transcription, just as it regulates nuclear gene transcription? (Figure 1) If such coupling exists, it would mean that, under the regulation of neural activity, materials and energy can be effectively coordinated and transformed, thereby supporting the transmission and storage of information. Using a mouse model, Professor Ma Huan's research group found that under conditions of enhanced neural activity, either through learning and memory tasks or artificially induced neural stimulation, mitochondrial gene transcription near the synapses of neurons was significantly increased.
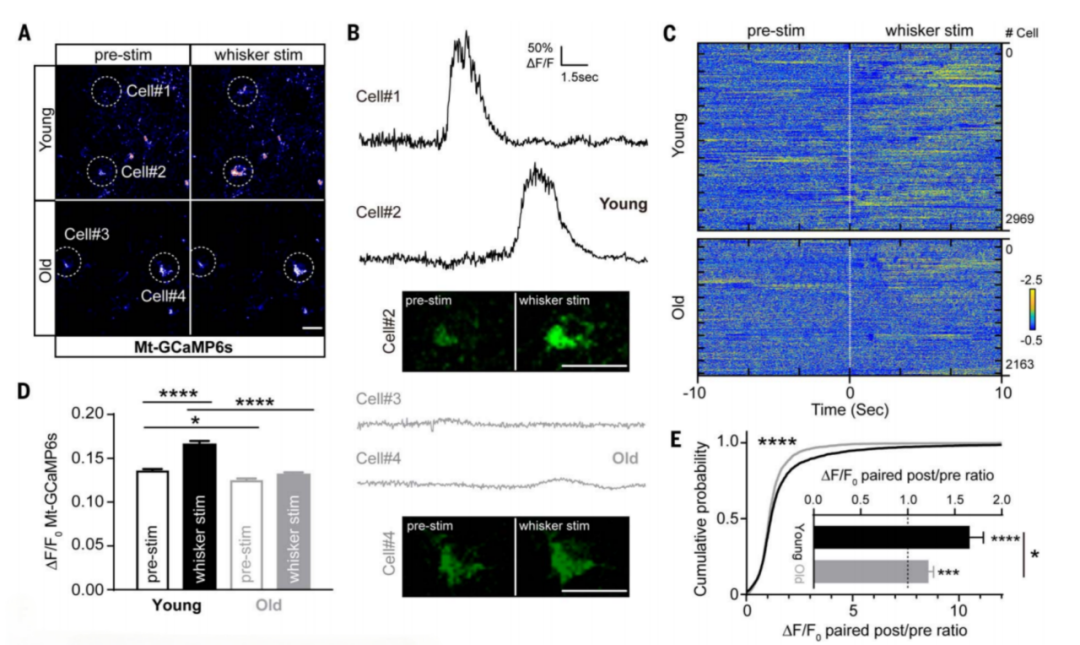
Figure 2: The role of mitochondrial calcium influx dependent on neural activity during aging and its key role in E-TCmito
Further studies showed that this coupling between neural activity and mitochondrial gene transcription heavily depends on the influx of calcium ions into mitochondria, which is induced by neural activity. This process is regulated by the calcium/calmodulin-dependent protein kinase II (CaMKII), located within the mitochondria. Once neural activity induces an increase in mitochondrial calcium ion concentration, the calcium-responsive transcription factor CREBmito within the mitochondria will respond and bind to the regulatory region of the mitochondrial genome, the D-loop, driving mitochondrial gene transcription (Figure 2). It is worth noting that both CaMKII and CREB are key proteins involved in neural activity-nuclear gene transcription, so discovering their distinct roles in mitochondria breaks the conventional textbook definitions of these two "star" signaling molecules. This finding reveals their multifaceted functions within the nervous system. Through a detailed analysis of the mechanisms of these key molecules, the research group has achieved precise regulation of neural activity-mitochondrial gene transcription at the molecular level. They have clarified that this process is crucial for mitochondrial biogenesis and quality control during neural activity, and the dynamic regulation of energy is foundational to maintaining synaptic function, homeostasis, and learning and memory during neural activity.
01Making the Brain "Younger" Through Thinking”
Current research shows that as the body ages, and especially when neurodegenerative diseases develop, the brain’s cognitive abilities and energy supply decline. The research team discovered that in such cases, the coupling between neuronal activity and mitochondrial gene transcription also weakens. "Therefore, we hypothesized that enhancing the neuronal activity-mitochondrial gene transcription coupling could improve brain function and combat cognitive aging," said Associate Researcher Li Wenwen. Gene manipulation in mouse brains supports this possibility. When the coupling between neuronal activity and mitochondrial gene transcription was inhibited in the mice, many age-related neurological pathologies, such as energy shortages and cognitive impairment, occurred. Based on this, the team designed several novel targeted molecular tools to precisely modify and enhance neuronal activity-mitochondrial gene transcription. The experiments showed that inhibiting this coupling impaired learning and memory in mice. However, long-term enhancement of this coupling mechanism significantly increased mitochondrial gene expression during learning and memory processes, improving the brain’s bioenergy supply and substantially enhancing cognitive function in the mice. "This provides a theoretical basis for resisting brain aging through 'more thinking,'" Li Wenwen noted. Understanding the basic molecular signal transduction mechanisms within neurons is crucial for understanding and controlling brain function. It not only offers a molecular framework for using "more thinking" to "counteract aging," but also presents a novel approach to combating cognitive aging. This research holds significant implications for understanding brain cognition and age-related neurodegenerative diseases. Clinical translation and drug development are underway, and preliminary results are encouraging.
02Low-Energy Information Processing in the Brain and Its Implications for AI
Elon Musk has pointed out that, in the development of artificial intelligence, the next constraint, beyond current chips, will be energy (electricity). Could the brain’s low-energy information processing provide insights for addressing the energy challenges faced by AI development? After elucidating the molecular mechanism of neuronal activity-mitochondrial gene coupling, the research team realized that this molecular mechanism, developed over long evolutionary periods, could be key to understanding the brain's efficient low-energy processing. Unlike other cells, neurons have a unique polarized structure—comprising the cell body, dendrites, and axons. The key to neuronal information processing and storage, the synapse, is located on the dendrites and axons, far from the cell body. These numerous distant synapses enable neurons to process information in parallel. However, they also create stringent demands for the local energy regulation at the synapses.
The research team’s findings suggest that, unlike traditional computers, which adopt an overall energy supply approach for information processing and storage, the mammalian brain uses a unique "on-demand energy supply" strategy. This involves deploying "energy packets" (mitochondria) near each data node (synapse) that can be regulated by information processing (neuronal activity). During information processing, mitochondria drive their gene transcription and protein synthesis via synaptic activity, realizing local, plastic energy supply regulation at the synapses. Revealing this fundamental signal coupling mechanism in living organisms could be the key to understanding how the brain efficiently processes complex information with low energy consumption. This insight provides a new direction for current artificial intelligence development, offering solutions to enhance information processing capabilities while reducing energy consumption.
Associate Researcher Li Wenwen and Ph.D. student Li Jiarui from Zhejiang University School of Medicine are the co-first authors of this paper. Professor Ma Huan from Zhejiang University Brain-Computer Intelligence National Key Laboratory, School of Brain Science and Brain Medicine, and Liangzhu Laboratory is the corresponding author. Additional authors include Professor Hu Hailan, Professor Li Tao, and others from the same university. The research also received support from academicians Duan Shumin and Chen Quan, and other scientists from Nankai University. The study was primarily funded by the National Natural Science Foundation of China, the Ministry of Science and Technology's Innovation 2030 "Brain Science and Brain-Like Research" major project, and other grants.
Original paper link:
https://www.science.org/doi/10.1126/science.adp6547
Professor Ma Huan is the Chair of the Academic Committee of the Zhejiang University School of Brain Science and Brain Medicine and a standing member of the Medical Department Academic Committee. He has published research in high-profile journals such as Science (2024), Cell (2014, 2012), Neuron (2021), Cell Reports (2022, 2021), Journal of Neuroscience (2023), and Nature Communications (2018). He has also been invited to write reviews and commentaries for Nature Reviews Neuroscience (2023) and Trends in Neuroscience (2024). Associate Researcher Li Wenwen completed her postdoctoral training in Professor Ma Huan's lab and has published several papers in Science and other journals. The lab is currently offering 3 postdoctoral positions, providing a research fund of 150,000 RMB for each new postdoc, along with competitive salaries ranging from 300,000 to 450,000 RMB. The lab atmosphere is collaborative, with well-established technology and full support for postdoc career development. Eleven former postdocs have secured permanent academic or research positions in universities, enterprises, or hospitals.
Email:
mah@zju.edu.cn;
Research Group Website:
https://person.zju.edu.cn/mah






